


Molluscum can finally be treated at home or on the go
Because parents have enough to worry about
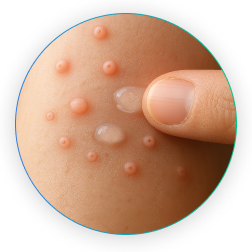
ZELSUVMI is the FIRST and ONLY FDA-approved at-home, prescription treatment for molluscum contagiosum in adults and children 1 year of age and older.
What is molluscum?
How can ZELSUVMI help treat molluscum?
What can I expect from treatment with ZELSUVMI?

Support to help you start and stay on treatment
Learn about resources, including copay assistance, to help on your treatment journey.

 Not an actual patient.
Not an actual patient.
What is ZELSUVMI?
ZELSUVMI is prescription medicine used on the skin (topical) to treat molluscum contagiosum in adults and children 1 year of age and older. It is not known if ZELSUVMI is safe and effective in children under 1 year of age.
IMPORTANT SAFETY INFORMATION
What warnings should I know about ZELSUVMI?
ZELSUVMI is for use on the skin (for topical use) only. Do not use ZELSUVMI near or in your eyes, mouth, or vagina.
ZELSUVMI may cause serious side effects, including:
- Application site reactions. Application site reactions, including allergic skin reactions, are common where ZELSUVMI is applied to your skin, but can also be severe. Stop using ZELSUVMI and tell your healthcare provider right away if you develop pain, burning, stinging, itching, swelling, or redness of your skin that lasts for more than 24 hours after treatment with ZELSUVMI.
Before using ZELSUVMI, tell your healthcare provider about all your medical conditions, including if you:
- Have other skin problems.
- Are pregnant or plan to become pregnant. It is not known if ZELSUVMI will harm your unborn baby.
- Are breastfeeding or plan to breastfeed. It is not known if ZELSUVMI passes into your breast milk. Talk to your healthcare provider about the best way to feed your baby during treatment with ZELSUVMI.
Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, and herbal supplements.
Keep ZELSUVMI and all medicines out of the reach of children.
What are the possible side effects of ZELSUVMI?
The most common side effects are application site reactions, including pain, burning, stinging, redness, itching, peeling or flaking, itchy dry skin rash, swelling, breakdown of the outer layer of the skin (erosion), lightening or darkening of the skin, blisters, irritation, and infection. These are not all of the possible side effects of ZELSUVMI.
You are encouraged to report side effects to FDA at 1-800-FDA-1088 or at www.fda.gov/medwatch. You may also report side effects and product complaints to Pelthos Inc. by calling 1-855-330-7546.
Please see the full Prescribing Information, Patient Information, and Instructions for Use for ZELSUVMI.




